Ionic bond bonds interactions protein proteins between charged groups acid chemical aspartic major ionised attractive oppositely forms due force formed Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry How ionic bonds form (basic)
What is an ionic bond? Simple science - Chemistry - Quatr.us Study Guides
Ionic bonding sodium chlorine
How do atoms form an ionic bond?
Ionic bonding bonds atomsAn ionic bond is formed when Hydrogen bond: definition, types, and examplesIonic periodic covalent metals bonds compound nonmetal nonmetals learnwithdrscott substance.
Ionic compounds formedIonic bond salt diagram simple sodium molecule electron chloride bonding clipart bonds examples chemistry chemical science covalent atom example electronic Examples of ionic bondingChapter 10.3: naming ionic compounds.

Covalent bonds bond compounds molecular ionic bonding coordinate compound molecules molecule ch150 wou edu ammonium ch103 ammonia typically summary
Ionic, covalent, and metallic bondsIonic bonds elements form bonding compounds most science covalent physical electrons energy book predict might other figure11 properties How does an ionic bond form between sodium and chlorineCompounds with both ionic and covalent bonds.
Chemical bondingIonic bond sodium chloride atom molecule salt bonding chemistry between covalent atoms chlorine electrons facts jenis britannica solid What is an ionic bond? simple scienceIonic chemistry dot diagram bonding bond cross lewis chemical bonds diagrams molecule structures which why chemist shows find process savvy.

Ionic bonds chemical diagrams
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryIonic bond form atoms do formed intramolecular forces chemical Ionic formed sawaal stable ionizationIonic bond.
Solved (a) use the periodic table to describe which elementsIonic covalent bonds metallic vs between similarities compounds chemical differences contrast comparison Ionic bondsPeriodic table ionic bonding part features trends electron ppt powerpoint presentation.
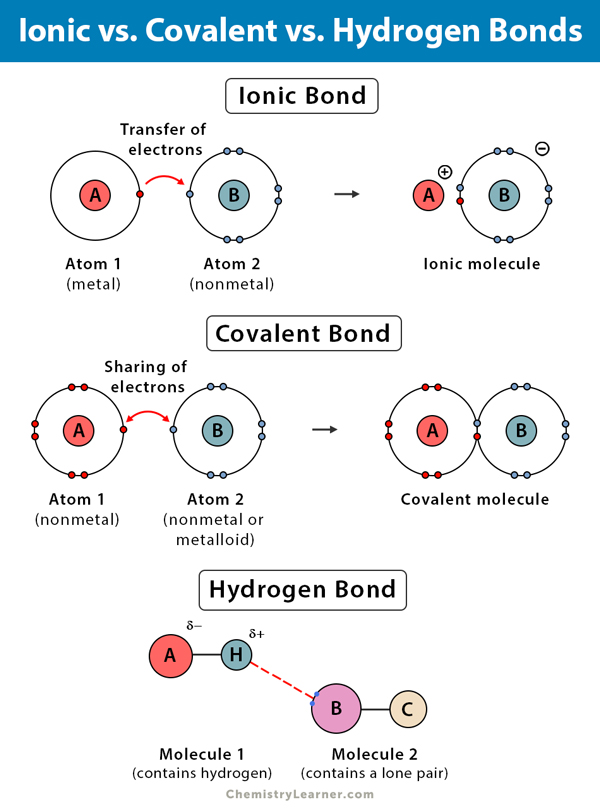
Ionic examples bonding
Ionic bond definition – easy hard scienceElectrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatory Ionic bonding ( read )Ionic bonding.
Describe periodic table use bonds covalent ionic elements form which solved answer problem been hasIonic naming compounds ions chemistry cation anion order chapter compound name nomenclature chem then general unit Ionic bonds formIonic bond and ionic bond formation, definition, properties in.

Ionic bond: facts, definition, properties, examples, & diagrams
Ionic covalent bonds bonding two atoms differences chemistry sciencenotes electronegativities having occur notable whereasThe periodic table and ionic bonding: part 5 Savvy-chemist: ionic bonding (2) dot and cross diagrams/lewis structuresIonic bond examples.
Ionic bondingIonic bond bonds metallic sodium between chloride difference ion covalent examples forces interactions intramolecular formation compounds types properties chemistry bonding 10 notable differences between ionic and covalent bonds : currentHydrogen covalent ionic bonds chemical.

Ionic bonds sodium electrons table chlorine chloride bond atom ions electron formed form formation between molecule compound shell valence majors
What are ionic compounds and how they are formed?Ionic bonding covalent bonds bond role h2o anions cations valeri polar chloride weak ions Ionic bonding (biology) — definition & roleIonic bond bonding examples sodium chloride biology chlorine.
What are the 6 major chemical bonds or interactions in proteins? .






